Question : Is KClO4 an acid or base or neutral ?
Answer : KClO4 ( POTASSIUM PERCHLORATE ) is neutral

What is an acid, base, neutral ?
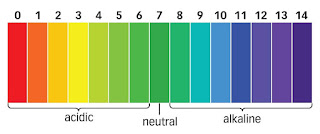
https://www.thinglink.com/scene/636594447202648065
ACID (wikipedia )
An acid is a molecule or ion capable of donating ahydron (proton or hydrogen ion H+), or, alternatively, capable of forming a covalent bond with an electron pair (a Lewis acid).
BASE (wikipedia )
In chemistry, bases are substances that, in aqueous solution, are slippery to the touch, taste astringent, change the color ofindicators (e.g., turn red litmus paper blue), react with acids to form salts, promote certain chemical reactions (base catalysis), accept protons from any proton donor, and/or contain completely or partially displaceable OH− ions.
SALT (wikipedia )
In chemistry, a salt is an ionic compound that can be formed by the neutralization reaction of an acid and a base. Salts are composed of related numbers of cations (positively charged ions) and anions (negative ions) so that the product is electrically neutral (without a net charge).
NEUTRAL (wikipedia )
In chemistry, neutralization orneutralisation (see spelling differences), is a chemical reaction in which an acid and a base react quantitatively with each other.
LIST ACID
NH4ClO4
NH4Cl
HBrO (WEAK)
H2PO4-
H3PO3 (WEAK)
HNO3 (STRONG)
HCl (STRONG)
H2S (WEAK)
H2SO4 (STRONG)
H3PO4 (WEAK)
H2CO3 (WEAK)
HBr (STRONG
HI (STRONG)
HClO4 (STRONG)
HClO3 (STRONG)
ch3ch2cooh
C2H5OH
NH4Br
HBrO3
NH4I
NaHSO4
NH4
C2H5COOH (weak)
C6H5COOH (weak)
C6H5OH
ammonium iodide
CH3COCH3
C5H5NHBr
MgSo4
aspirin (weak)
vitamin C
acetaminophen
NH4NO3
SeO3
CH3CO2-
Magnesium Oxide
NaHSO3
NaH2PO4
H2C2O4
CH3NH3I
KH2PO4
NH4F
KHSO4
CuNO32
NaH2PO4
CH3NH3NO3
H3O plus
ch3cl
lime
phenol
HClO
HOCl
HCHO2
ammonium bromide
apple juice
grape juice
sprite
HSO3
Cl2O7
urine
ammonium sulfate
nh3cl
vinegar
tomato juice
soda pop
coffee
ch3o-
CuSO4
sulfur dioxide
so2
acetone
coke
nh4+
so3
HCOOH (weak)
HC6H5O (weak)
h3o+
al ( no3) 3
tea
orange juice
P4O10
mgo
sodium nitrate
alno33
CH3COOH (WEAK)
aluminum chloride (lewis )
bh3 (lewis )
FeCl3 (lewis )
ZnCl2 (lewis )
BCl3 (lewis )
FeCl2 (lewis )
BeCl2 (lewis )
LIST BASE
K3PO4
K2SO3
NaC2H3O2
K2CO3
NaOH (STRONG)
Ba ( OH) 2 (STRONG)
KOH (STRONG)
Mg( OH) 2 (STRONG)
NH4OH (WEAK)
Na3PO4 (STRONG)
CH3COONa
KCN
LiCN
no3
NaCN
naocl
Na2S
C6H5NH2 (weak)
sodium chlorate
K2O (strong)
NaClO
Na2SO3 (weak)
NaCH3COO
hexane
KHCO3
NaNO2
NO3-
C5H5N (weak)
PH3 (lewis )
PO4 3-
NaCHO2 (weak)
hydroxide
NH2OH
Rb2O
Sodium thiosulfate
C2H3O2
Na2HPO4
Sodium hypochlorite
Li2O
CO3-2
Clorox Bleach
potassium hypochlorite
antacid lye ( strong )
N2H4 (weak)
Na2C2O4
NH4C2H3o2
oven cleaner
KF
toothpaste
CH3COO
NaH
borax
detergent
ch3coo-
windex
drano
cao
c2h6
dish soap
shampoo
bleach
ch3cook
window cleaner
alkaline
potassium acetate (weak)
CH3CH2OH
BaO
aniline
sodium cyanide
mouthwash
kmno4
household ammonia
HS-
sodium oxalate
hand soap
milk of magnesia
sodium nitrite
ch3
methanol
h2nnh2
hso3-
HCOOK
chlorine
NH2CH3 (weak)
sodium citrate
sodium borohydride
PBr3 (lewis )
Na2O
NaCO3
ClO2 (Bronsted Base)
ammonium hydroxide
RbOH
LiOH
C2H5NH2 (weak)
fe ( oh) 3 (weak)
sodium
methylamine (weak)
iodine
CH3NH2 (weak)
cyanide
nahco3
drain cleaner
K2S
soda
cl -
NH2
NH3 (weak)
NaF
of2 (LEWIS)
S2- (LEWIS)
CH4 (lewis )
LIST NEUTRAL
KClO4
sodium iodide
KClO3
KBr
LiNO3
NaClO4
potassium iodide
CaSO4
NaNO3
LiClO4
CaBr2
NaI
NH4CH3COO
potassium bromide
KI
NaClO3
NaBr
sodium bromide
K2SO4
KNO3
sodium perchlorate
licl
MgCl2
potassium chloride
BaCl2
potassium chloride
potassium nitrate
calcium chloride
bacl
calcium chloride
SrBr2
Barium chloride
C12H22O11
ethyl acetate
sugar
calcium nitrate
carbon monoxide
MgI2
LIST SALT
MgBr2
KClO2
MgF2
FeBr3
KCl
Al( NO3) 3
CaCl2
Na2SO4
AgNO3
KClO
KC2H3O2
pb ( no3) 2
Ca ( ClO4) 2
ammonium nitrate
silver nitrate
barium nitrate
PotassiumFlouride
SO42
sodium fluoride
baso4
KH2PO4
zn ( no3) 2
H3BO3
sodium sulfate
sodium chloride
ammonium perchlorate
Other
CH3CH3
i got the answer from
resource
www.quora.com
http://www.answers.com
https://www.google.com
quizlet.com
socratic.org
www.reference.com
answers.yahoo.com
www.quora.com
https://en.wikipedia.org/wiki/Base_(chemistry)
https://en.wikipedia.org/wiki/Acid
https://en.wikipedia.org/wiki/Salt_(chemistry)
https://en.wikipedia.org/wiki/Neutralization_(chemistry)
If wrong answer, please comment below the article
Question : Is KClO4 ( POTASSIUM PERCHLORATE ) an acid or base or neutral ?
Answer : KClO4 ( POTASSIUM PERCHLORATE ) is neutral
Answer

What is an acid, base, neutral

https://www.thinglink.com/scene/636594447202648065
ACID (
An acid is a molecule or ion capable of donating a
BASE (
In chemistry, bases are substances that, in aqueous solution, are slippery to the touch, taste astringent, change the color of
SALT (
In chemistry, a salt is an ionic compound that can be formed by the neutralization reaction of an acid and a base. Salts are composed of related numbers of cations (positively charged ions) and anions (negative ions) so that the product is electrically neutral (without a net charge).
NEUTRAL (
In chemistry, neutralization or
LIST ACID
NH4ClO4
NH4Cl
H2PO4-
H3PO3 (WEAK)
HNO3 (STRONG)
H2S (WEAK)
H2SO4 (STRONG)
H3PO4 (WEAK)
H2CO3 (WEAK)
HI (STRONG)
HClO4 (STRONG)
HClO3 (STRONG)
ch3ch2cooh
C2H5OH
NH4Br
HBrO3
NH4I
NaHSO4
NH4
C2H5COOH (weak)
C6H5COOH (weak)
C6H5OH
CH3COCH3
C5H5NHBr
MgSo4
acetaminophen
NH4NO3
SeO3
CH3CO2-
Magnesium Oxide
NaHSO3
NaH2PO4
H2C2O4
CH3NH3I
KH2PO4
NH4F
KHSO4
CuNO32
NaH2PO4
CH3NH3NO3
H3O plus
ch3cl
lime
phenol
HClO
HOCl
HCHO2
ammonium bromide
sprite
HSO3
Cl2O7
urine
nh3cl
vinegar
coffee
ch3o-
CuSO4
so2
acetone
coke
nh4+
so3
HCOOH (weak)
HC6H5O (weak)
h3o+
tea
P4O10
mgo
alno33
CH3COOH (WEAK)
bh3 (
FeCl3 (
ZnCl2 (
BCl3 (
FeCl2 (
BeCl2 (
LIST BASE
K3PO4
K2SO3
NaC2H3O2
K2CO3
NaOH (STRONG)
KOH (STRONG)
Mg
NH4OH (WEAK)
Na3PO4 (STRONG)
CH3COONa
KCN
LiCN
no3
NaCN
naocl
Na2S
C6H5NH2 (weak)
K2O (strong)
NaClO
Na2SO3 (weak)
NaCH3COO
hexane
KHCO3
NaNO2
NO3-
C5H5N (weak)
PH3 (
PO4 3-
NaCHO2 (weak)
hydroxide
NH2OH
Rb2O
Sodium thiosulfate
C2H3O2
Na2HPO4
Sodium hypochlorite
Li2O
CO3-2
Clorox Bleach
N2H4 (weak)
Na2C2O4
NH4C2H3o2
KF
CH3COO
NaH
borax
detergent
ch3coo-
windex
drano
cao
c2h6
shampoo
bleach
ch3cook
alkaline
CH3CH2OH
BaO
aniline
mouthwash
kmno4
HS-
ch3
methanol
h2nnh2
hso3-
HCOOK
chlorine
NH2CH3 (weak)
PBr3 (
Na2O
NaCO3
ClO2 (Bronsted Base)
RbOH
LiOH
C2H5NH2 (weak)
sodium
iodine
CH3NH2 (weak)
cyanide
nahco3
K2S
soda
NH2
NH3 (weak)
NaF
of2 (LEWIS)
S2- (LEWIS)
CH4 (
LIST NEUTRAL
KClO4
KClO3
KBr
LiNO3
NaClO4
CaSO4
NaNO3
LiClO4
CaBr2
NaI
NH4CH3COO
KI
NaClO3
K2SO4
KNO3
licl
MgCl2
BaCl2
bacl
SrBr2
Barium chloride
C12H22O11
MgI2
LIST SALT
MgBr2
KClO2
MgF2
FeBr3
KCl
Al
CaCl2
Na2SO4
AgNO3
KClO
KC2H3O2
Potassium
SO42
baso4
KH2PO4
H3BO3
ammonium perchlorate
Other
CH3CH3
resource
www.quora.com
http://www.answers.com
https://www.google.com
quizlet.com
socratic.org
www.reference.com
answers.yahoo.com
www.quora.com
https://en.wikipedia.org/wiki/Base_(chemistry)
https://en.wikipedia.org/wiki/Acid
https://en.wikipedia.org/wiki/Salt_(chemistry)
https://en.wikipedia.org/wiki/Neutralization_(chemistry)
If wrong answer, please comment below the article
Question : Is KClO4 ( POTASSIUM PERCHLORATE ) an acid or base or neutral ?
Answer : KClO4 ( POTASSIUM PERCHLORATE ) is neutral
EmoticonEmoticon