Question = Is I2 ( Iodine ) polar or nonpolar ?
Answer = I2 ( Iodine ) is Nonpolar

What is polar and non-polar?
Polar
"In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. A polar molecule with two or more polar bonds must have an asymmetric geometry so that the bond dipoles do not cancel each other.
Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points." (Wikipedia)
Polar molecules
A polar molecule has a net dipole as a result of the opposing charges (i.e. having partial positive and partial negative charges) from polar bonds arranged asymmetrically. (Wikipedia)
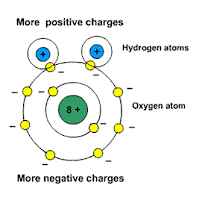
http://www.school-for-champions.com
Picture: water is a polar molecule
Example polar molecules
Ammonia (NH3)
Sulfur Dioxide (SO2)
Hydrogen Sulfide (H2S)
Nonpolar molecules
A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. (Wikipedia)
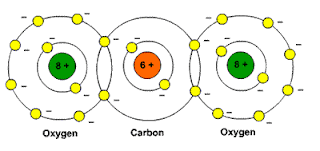
http://www.school-for-champions.com
Picture: Carbon dioxide
Example molecules non polar
Toluene
Gasoline
Helium (He)
Neon (Ne)
Krypton (Kr)
Xenon (Xe)
Hydrogen (H2)
Nitrogen (N2)
Oxygen (O2)
Carbon Dioxide (CO2)
Methane (CH4)
Ethylene (C2H4)
List molecules polar and non polar
Molecules polar
SCN- ( Thiocyanate )
HCO3- (Bicarbonate)
BrCl3 (Bromine Trichloride)
HCO3-1
AsCl3 (Trichloroarsine)
OCl2
NO2Cl (Nitryl chloride)
CH3F (Fluoromethane)
H3O+ Hydronium
ClF (Chlorine monofluoride)
ClF3 (Chlorine trifluoride)
CF2Cl2 (Dichlorodifluoromethane)
SeF4
CH3OCH3 (Dimethyl ether)
NH2-
CH3NH2 (Methylamine)
CH2Br2 (Dibromomethane)
OH2 (Hydroxide)
C2Cl2
NO+ (nitrilooxonium)
SBr2
ICl4+
NO
SCl6
NOBr (Nitrosyl bromide)
ICl3 (Iodine trichloride)
CH2F2 (Difluoromethane)
SeH2 (Hydrogen selenide)
COS (Cobalt sulfide)
H2SO4 (SULFURIC ACID)
H2CO ( Formaldehyde )
NF3 (NITROGEN TRIFLUORIDE)
C2H2Br2 (Acetylene dibromide)
TeF4
SCN
CLO3- (Chlorate)
ICl5
urea
SO2Cl2 (Sulfuryl chloride)
H2Se (Hydrogen selenide)
NH2
SbF3
AsF3 (ARSENIC TRIFLUORIDE)
BrF
IF3 (Iodine trifluoride)
SH2
SCl4
CO (Carbon monoxide)
H3O
N2H2
CH3COOH (acetic acid)
salt
SeO2
nitrogen trifluoride
CCl2F2
N2H4
C2H5OH
NoCl (NITROSYL CHLORIDE)
C2H6O (Ethanol-d6)
SOCl2
H3O+ (Hydronium)
CHF3 (Fluoroform)
NaCl (sodium chloride)
AsH3 (Arsine)
NH2Cl
OCS (Carbonyl sulfide)
SiCl2F2
glucose
CH3
PoCl3 (PHOSPHORUS OXYCHLORIDE)
MgCl2
vinegar
IOF5
phosphate
CHBr3 (Bromoform)
ICl (IODINE MONOCHLORIDE)
carbon monoxide
sulfur dioxide
SF2 (Sulfur difluoride)
NH3 (ammonia)
SO2 (sulfur dioxide)
SCl2 (Sulfur dichloride)
PH3 (Phosphine)
CH2O (Formaldehyde)
HBr (HYDROBROMIC ACID)
NCl3 (Nitrogen trichloride)
PF3 (Phosphorus trifluoride)
ethyl acetate
NO3- (nitrate)
NO2 (Nitrogen dioxide)
H2O2 (hydrogen peroxide)
CH3Br (Bromomethane)
O3 (OZONE)
ammonia
OH- (Hydroxide)
COCl2 (Cobalt(II) chloride)
glycerol
oh
chloroform
nitrogen trichloride
benzoic acid
ether
no3
CLF5
methylene chloride
sodium chloride
CH3SH
sodium acetate
1-butanol
Clo-
C4H10
libr
cabr2 (Calcium bromide)
CH3CH2CH2OH
LiNO3 (Lithium nitrate)
MgO
SeOBr2
clo3
c3h7oh
glycerine
bri5
BrO2
ammonium chloride
CF3Cl
BF2Cl
IBr
GaCl3
KMnO4 (POTASSIUM PERMANGANATE)
Na2S (sodium sulfide)
KOH (POTASSIUM HYDROXIDE)
HCOOH
carbohydrates
ClO4
Cl2CO
clo2-
succinic acid
H2SO3 (Sulfurous acid)
sodium nitrate
ethyne
NO2F
CCL3F (Fluorotrichloromethane)
ammonium
ibr3
fluoromethane
C2H5Cl
TeCl4
2-propanol
starch
ch2cl
XeOF4
SnCl2
SCO (Carbonyl sulfide)
SbBr3
H2CS
HC2H3O2
po(oh)3
Propanol
1-propanol
bro3-
ch3ch2ch2ch3
CF2Br2
acetic acid
aniline
CF
chlorine trifluoride
phenol
clo2
potassium permanganate
CH2S
nitrogen monoxide
ch3cho
glass
SH
COH2
SOF4
DNA
CH3CH2NH2
Malonic Acid
ethylene glycol
hydrogen bromide
SeCl4
hydrogen peroxide
ozone
formaldehyde
po3 3
difluoromethane
CHCl2
rubbing alcohol
fluorene or fluorenol
vitamin c
nitrate
dimethyl ether
SCl
if4+
IF5 ( Iodine pentafluoride )
C3H6O or (ch3)2co or ch3coch3 ( acetone )
CH4O ( Methanol )
CH3CH2OH ( Ethanol )
CH2Cl2 ( DICHLOROMETHANE )
H2S ( hydrogen sulfide )
HF ( Hydrogen fluoride )
NBr3 ( Nitrogen tribromide )
Cl2O ( dichlorine monoxide )
OF2 ( Oxygen difluoride )
XeO3 ( xenon trioxide )
HCl ( hydrogen chloride )
CH3OH ( Methanol or methyl alcohol )
BrF5 ( BROMINE PENTAFLUORIDE )
BrF3 ( BROMINE TRIFLUORIDE )
CH3CN ( ACETONITRILE )
CH3CL or H3CCl (Chloromethane)
PCl3 ( PHOSPHORUS TRICHLORIDE )
cn- ( cyanide )
CaCO3 ( Calcium carbonate )
SF4 ( sulfur tetrafluoride )
PBr3 ( PHOSPHORUS TRIBROMIDE )
HClO ( HYPOCHLOROUS ACID )
So3 2- ( sulfite ion )
NO2- ( nitrite )
isopropyl alcohol ( C3H8O )
KBr ( POTASSIUM BROMIDE )
PCl5 ( PHOSPHORUS PENTACHLORIDE )
Na2SO4 ( sodium sulfate )
nai ( Sodium iodide )
CaCl2 ( CALCIUM CHLORIDE )
NH4NO3 ( Ammonium nitrate )
HI ( Hydrogen iodide )
C6H12O6 ( dextrose )
HNO3 ( NITRIC ACID )
CHCl3 (CHLOROFORM or trichloromethane)
aspirin or acetylsalicylic acid
NI3 ( Nitrogen triiodide )
N2O ( Nitrous oxide )
H2O ( water )
HCN ( Hydrogen cyanide )
cl2xef2
triphenylmethanol ( C19H16O )
COF2 ( Carbonyl fluoride )
SeOF2
teo2 ( Tellurium dioxide )
c2h6o2 ( Ethylene glycol )
C2H2Cl2
BFCl2
SeCl2
NiCl2 ( Nickel(II) chloride )
S2CL2 ( Disulfur dichloride )
TeCl2 ( Tellurium dichloride )
SF4Cl2
BaCl2 ( Barium chloride )
SiH2Cl2
SeBr2 ( Selenium(II) Bromide )
OBr2
XeO2F2
SeF2
PCl3F2
CF2H2
sugar ( sucrose )
tyrosine
Serine
Arginine
Asparagine
Aspartate
Glutamate
Glutamine
Histidine
Lysine
Threonine
NaOH ( Sodium hydroxide )
NH2Br
Glutamic Acid
caffeine
dinitrogen monoxide
aspartic acid
Si-Br
hclo4
HOCl
n-h
CH3CH2Cl ( Chloroethane )
BrF4
H3PO4 ( Phosphoric acid )
H-O
2-propanol
SF5
Aldehyde
citric acid
NH4Cl ( Ammonium chloride )
pentanol
acetanilide
carbonyl
SH
NaHCO3 ( sodium bicarbonate )
pcl4f
acetophenone
benzil
cellulose
ch2chch3
dodecyl alcohol
sodium benzoate
N2O5 ( Dinitrogen pentoxide )
hcho
ascorbic acid
SF5Cl
c12h22o11 ( sucrose )
SeS2 ( Selenium disulfide )
epinephrine
ammonium acetate
H2Te
Carboxyl acid
fructose
FCL
Hydroxyl
Salicylic acid
N2O3 ( Dinitrogen trioxide )
Sulfhydryl
ch2oh
OCl
amphetamine
sihcl3
AgNO3 ( Silver nitrate )
NH2OH
peptide bond
lauric acid
CH3COH
H2CO3 ( carbonic acid )
ester
SI4
ch3chohch3 ( isopropanol )
SiF6 2-
C2H3Cl
Al2o3
HOH
hco2h ( formic acid )
Molecules non polar
HBrO ( Hypobromous acid )
SiF4 (Silicon tetrafluoride)
CH3CH3 (Ethane)
N2 (Nitrogen)
CO3 2- (Carbonate)
Heptane-
XeO4 (Xenon tetroxide)
CH3I (Methyl iodide)
CBr4 (Carbon tetrabromide)
AlCl3 (Aluminum trichloride)
NH4Br
C2H4Cl2 (dichloroethane)
ligroin
SO4 2- (Sulfate)
SF6 (Sulfur hexafluoride)
C2F2 ( Ethyne )
PO4 3- (phosphate)
GeH4 (Germane)
PBr5 (Phosphorus pentabromide)
NH4 (ammonium)
wax
CI4 (Tetraiodomethane)
bbr3 (BORON TRIBROMIDE)
IF4-
BeF2 (Beryllium difluoride)
GaH3 (Gallane)
SeBr4 (Selenium tetrabromide)
KrF4
C2H6 (Ethane)
O2 (Oxygen)
biphenyl
PO4 3
P4 (Phosphorus tetramer)
SO4 2-
naphthalene
XeCl2
BrCl5
SeF6
SeO3
SiH4 (silane)
carbon tetrabromide
AsF5 (Arsenic pentafluoride)
BeH2 (Beryllium hydride)
CH2
cholesterol
pentane
ClF2
C3H8
Propane
cyclohexane
chlorine
PF5 (Pentafluorophosphorane)
BrCl
CO2 (carbon dioxide)
CCl4 (CARBON TETRACHLORIDE)
XeF2
CS2 (CARBON DISULFIDE)
SO3 (SULFUR TRIOXIDE)
XeF4 (Xenon tetrafluoride)
BCl3 (BORON TRICHLORIDE)
BeCl2 (Beryllium dichloride)
CF4 (CARBON TETRAFLUORIDE)
Cl2 (Chlorine)
i3-
carbon tetrachloride
carbon disulfide
methane
glycine
sulfur trioxide
benzophenone
oxygen
AlF3 (Aluminum fluoride)
beryllium dichloride
C8H18 (octane)
C2Cl4
PF6-
XeCl4
SbF5 (ANTIMONY PENTAFLUORIDE)
CH3CH2CH3
C5H12 (PENTANE)
silane
s8
BF4
BF4-
SeCl6
BeBr2
BeI2
CSe2
Pcl4
C3H6
AlH3 (Aluminum trihydride)
TeO3 (Tellurium trioxide)
Br-Br
AsCl5
octane
carbonate ions
ClF4 plus
acids
kerosene
acetylene
C-C
ethane
CCl
Cl4
cbr
sulfate
butter
triglycerides
grease
beryllium chloride
carbon tetrafluoride
butane
silicon tetrafluoride
nitrogen
ICl2-
bromine
hydrocarbon
HE
boron trichloride
CS
sulfur hexafluoride
sis2
xenon tetrafluoride
CH4
vitamin e
Br2 (Bromine)
KNO3 ( Potassium nitrate )
C6H14 or CH3(CH2)4CH3 ( HEXANE )
C6H6 ( benzene )
I2 ( Iodine )
H2 ( Hydrogen )
SiO2 ( silica gel or silicon dioxide )
SiCl4 ( Silicon tetrachloride )
C2H4 ( Ethylene )
C2H2 (Ethyne)
KrF2 ( krypton difluoride )
toluene ( C7H8 )
F2 ( Fluorine )
SiBr4 ( silicon tetrabromide )
BH3 ( Borane )
BF3 ( Boron trifluoride )
xeo2 ( Xenon dioxide )
TeO3 ( Tellurium trioxide )
bcl2
PbCl2 ( Lead(II) chloride )
HgCl2 ( Mercury(II) chloride )
C2Br2 ( Dibromoacetylene )
CI2 or cl-cl ( Chlorine )
scl4i2
ch2i2 ( Diiodomethane )
if2-
n2f2
scl4f2
oil
Lipids
ICl4-
c ( carbon )
diethyl ether ( (C2H5)2O or CH3CH2OCH2CH3 )
soap
tryptophan
Proline
Phenylalanine
Alanine
Cysteine
Isoleucine
Leucine
Methionine
Valine
paraffin wax
C-H ( carbon hydrogen )
vegetable oil
Gasoline
H-H
F-F
FCN ( Cyanogen fluoride )
ClCN ( CYANOGEN CHLORIDE )
AlBr3 ( ALUMINUM BROMIDE )
(CH3CH2)2O
Petroleum Ether
C2F6
stearic acid
1-decanol
octahedral
C6H12
ch3ch2ch2ch2ch3
square planar
Helium ( He )
ibuprofen
propene
hcch
PF2Cl3
C3H4
Na2CO3 ( sodium carbonate )
phosphorus triodide ( PI3 )
C4H8 ( Isobutene )
vitamin a
SbCl5 ( Antimony pentachloride )
polyethylene
SnCl4 ( Tin(IV) chloride )
graphite
FeCl3 ( Iron (III) Chloride )
polystyrene
C2Cl6
decane
Sii4 ( Silicon tetraiodide )
boron trihydride
C2F4 ( Tetrafluoroethylene )
octanol
teflon
Ionic
NaBr
NaF
kf
NaNO3
cao
ki
lif
licl (LITHIUM CHLORIDE)
ammonium sulfate
MgF2
ki & lif
KCl ( Potassium Chloride )
MgBr2 ( Magnesium bromide )
CaF2 ( Calcium fluoride )
Na2O ( Sodium Oxide )
CsCl ( Cesium Chloride )
MgSO4 ( Magnesium Sulfate )
CuSO4 ( Copper (II) Sulfate )
CsI ( Cesium Iodide )
RbCl ( Rubidium chloride )
We get answers from
Resources:
https://en.wikipedia.org/wiki/Chemical_polarity
http://www.school-for-champions.com/chemistry/polar_molecules.htm#.WZIGddJJbcc
reference.com
www.quora.com
answers.yahoo.com
answers.com
youtube.com
google.com
https://pubchem.ncbi.nlm.nih.gov
If the answer is wrong, please comment in below article !
Question = Is I2 ( Iodine ) polar or nonpolar ?
Answer = I2 ( Iodine ) is Nonpolar
Answer = I2 ( Iodine ) is Nonpolar

What is polar and non-polar?
Polar
"In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. A polar molecule with two or more polar bonds must have an asymmetric geometry so that the bond dipoles do not cancel each other.
Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points." (Wikipedia)
Polar molecules
A polar molecule has a net dipole as a result of the opposing charges (i.e. having partial positive and partial negative charges) from polar bonds arranged asymmetrically. (Wikipedia)
http://www.school-for-champions.com
Picture: water is a polar molecule
Example polar molecules
Ammonia (NH3)
Sulfur Dioxide (SO2)
Hydrogen Sulfide (H2S)
Nonpolar molecules
A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. (Wikipedia)

http://www.school-for-champions.com
Picture: Carbon dioxide
Example molecules non polar
Toluene
Gasoline
Helium (He)
Neon (Ne)
Krypton (Kr)
Xenon (Xe)
Hydrogen (H2)
Nitrogen (N2)
Oxygen (O2)
Carbon Dioxide (CO2)
Methane (CH4)
Ethylene (C2H4)
List molecules polar and non polar
Molecules polar
SCN- ( Thiocyanate )
HCO3- (Bicarbonate)
BrCl3 (Bromine Trichloride)
HCO3-1
AsCl3 (Trichloroarsine)
OCl2
NO2Cl (Nitryl chloride)
CH3F (Fluoromethane)
H3O+ Hydronium
ClF (Chlorine monofluoride)
ClF3 (Chlorine trifluoride)
CF2Cl2 (Dichlorodifluoromethane)
SeF4
CH3OCH3 (Dimethyl ether)
NH2-
CH3NH2 (Methylamine)
CH2Br2 (Dibromomethane)
OH2 (Hydroxide)
C2Cl2
NO+ (nitrilooxonium)
SBr2
ICl4+
NO
SCl6
NOBr (Nitrosyl bromide)
ICl3 (Iodine trichloride)
CH2F2 (Difluoromethane)
SeH2 (Hydrogen selenide)
COS (Cobalt sulfide)
H2SO4 (SULFURIC ACID)
H2CO ( Formaldehyde )
NF3 (NITROGEN TRIFLUORIDE)
C2H2Br2 (Acetylene dibromide)
TeF4
SCN
CLO3- (Chlorate)
ICl5
urea
SO2Cl2 (Sulfuryl chloride)
H2Se (Hydrogen selenide)
NH2
SbF3
AsF3 (ARSENIC TRIFLUORIDE)
BrF
IF3 (Iodine trifluoride)
SH2
SCl4
CO (Carbon monoxide)
H3O
N2H2
CH3COOH (acetic acid)
salt
SeO2
nitrogen trifluoride
CCl2F2
N2H4
C2H5OH
NoCl (NITROSYL CHLORIDE)
C2H6O (Ethanol-d6)
SOCl2
H3O+ (Hydronium)
CHF3 (Fluoroform)
NaCl (sodium chloride)
AsH3 (Arsine)
NH2Cl
OCS (Carbonyl sulfide)
SiCl2F2
glucose
CH3
PoCl3 (PHOSPHORUS OXYCHLORIDE)
MgCl2
vinegar
IOF5
phosphate
CHBr3 (Bromoform)
ICl (IODINE MONOCHLORIDE)
carbon monoxide
sulfur dioxide
SF2 (Sulfur difluoride)
NH3 (ammonia)
SO2 (sulfur dioxide)
SCl2 (Sulfur dichloride)
PH3 (Phosphine)
CH2O (Formaldehyde)
HBr (HYDROBROMIC ACID)
NCl3 (Nitrogen trichloride)
PF3 (Phosphorus trifluoride)
ethyl acetate
NO3- (nitrate)
NO2 (Nitrogen dioxide)
H2O2 (hydrogen peroxide)
CH3Br (Bromomethane)
O3 (OZONE)
ammonia
OH- (Hydroxide)
COCl2 (Cobalt(II) chloride)
glycerol
oh
chloroform
nitrogen trichloride
benzoic acid
ether
no3
CLF5
methylene chloride
sodium chloride
CH3SH
sodium acetate
1-butanol
Clo-
C4H10
libr
cabr2 (Calcium bromide)
CH3CH2CH2OH
LiNO3 (Lithium nitrate)
MgO
SeOBr2
clo3
c3h7oh
glycerine
bri5
BrO2
ammonium chloride
CF3Cl
BF2Cl
IBr
GaCl3
KMnO4 (POTASSIUM PERMANGANATE)
Na2S (sodium sulfide)
KOH (POTASSIUM HYDROXIDE)
HCOOH
carbohydrates
ClO4
Cl2CO
clo2-
succinic acid
H2SO3 (Sulfurous acid)
sodium nitrate
ethyne
NO2F
CCL3F (Fluorotrichloromethane)
ammonium
ibr3
fluoromethane
C2H5Cl
TeCl4
2-propanol
starch
ch2cl
XeOF4
SnCl2
SCO (Carbonyl sulfide)
SbBr3
H2CS
HC2H3O2
po(oh)3
Propanol
1-propanol
bro3-
ch3ch2ch2ch3
CF2Br2
acetic acid
aniline
CF
chlorine trifluoride
phenol
clo2
potassium permanganate
CH2S
nitrogen monoxide
ch3cho
glass
SH
COH2
SOF4
DNA
CH3CH2NH2
Malonic Acid
ethylene glycol
hydrogen bromide
SeCl4
hydrogen peroxide
ozone
formaldehyde
po3 3
difluoromethane
CHCl2
rubbing alcohol
fluorene or fluorenol
vitamin c
nitrate
dimethyl ether
SCl
if4+
IF5 ( Iodine pentafluoride )
C3H6O or (ch3)2co or ch3coch3 ( acetone )
CH4O ( Methanol )
CH3CH2OH ( Ethanol )
CH2Cl2 ( DICHLOROMETHANE )
H2S ( hydrogen sulfide )
HF ( Hydrogen fluoride )
NBr3 ( Nitrogen tribromide )
Cl2O ( dichlorine monoxide )
OF2 ( Oxygen difluoride )
XeO3 ( xenon trioxide )
HCl ( hydrogen chloride )
CH3OH ( Methanol or methyl alcohol )
BrF5 ( BROMINE PENTAFLUORIDE )
BrF3 ( BROMINE TRIFLUORIDE )
CH3CN ( ACETONITRILE )
CH3CL or H3CCl (Chloromethane)
PCl3 ( PHOSPHORUS TRICHLORIDE )
cn- ( cyanide )
CaCO3 ( Calcium carbonate )
SF4 ( sulfur tetrafluoride )
PBr3 ( PHOSPHORUS TRIBROMIDE )
HClO ( HYPOCHLOROUS ACID )
So3 2- ( sulfite ion )
NO2- ( nitrite )
isopropyl alcohol ( C3H8O )
KBr ( POTASSIUM BROMIDE )
PCl5 ( PHOSPHORUS PENTACHLORIDE )
Na2SO4 ( sodium sulfate )
nai ( Sodium iodide )
CaCl2 ( CALCIUM CHLORIDE )
NH4NO3 ( Ammonium nitrate )
HI ( Hydrogen iodide )
C6H12O6 ( dextrose )
HNO3 ( NITRIC ACID )
CHCl3 (CHLOROFORM or trichloromethane)
aspirin or acetylsalicylic acid
NI3 ( Nitrogen triiodide )
N2O ( Nitrous oxide )
H2O ( water )
HCN ( Hydrogen cyanide )
cl2xef2
triphenylmethanol ( C19H16O )
COF2 ( Carbonyl fluoride )
SeOF2
teo2 ( Tellurium dioxide )
c2h6o2 ( Ethylene glycol )
C2H2Cl2
BFCl2
SeCl2
NiCl2 ( Nickel(II) chloride )
S2CL2 ( Disulfur dichloride )
TeCl2 ( Tellurium dichloride )
SF4Cl2
BaCl2 ( Barium chloride )
SiH2Cl2
SeBr2 ( Selenium(II) Bromide )
OBr2
XeO2F2
SeF2
PCl3F2
CF2H2
sugar ( sucrose )
tyrosine
Serine
Arginine
Asparagine
Aspartate
Glutamate
Glutamine
Histidine
Lysine
Threonine
NaOH ( Sodium hydroxide )
NH2Br
Glutamic Acid
caffeine
dinitrogen monoxide
aspartic acid
Si-Br
hclo4
HOCl
n-h
CH3CH2Cl ( Chloroethane )
BrF4
H3PO4 ( Phosphoric acid )
H-O
2-propanol
SF5
Aldehyde
citric acid
NH4Cl ( Ammonium chloride )
pentanol
acetanilide
carbonyl
SH
NaHCO3 ( sodium bicarbonate )
pcl4f
acetophenone
benzil
cellulose
ch2chch3
dodecyl alcohol
sodium benzoate
N2O5 ( Dinitrogen pentoxide )
hcho
ascorbic acid
SF5Cl
c12h22o11 ( sucrose )
SeS2 ( Selenium disulfide )
epinephrine
ammonium acetate
H2Te
Carboxyl acid
fructose
FCL
Hydroxyl
Salicylic acid
N2O3 ( Dinitrogen trioxide )
Sulfhydryl
ch2oh
OCl
amphetamine
sihcl3
AgNO3 ( Silver nitrate )
NH2OH
peptide bond
lauric acid
CH3COH
H2CO3 ( carbonic acid )
ester
SI4
ch3chohch3 ( isopropanol )
SiF6 2-
C2H3Cl
Al2o3
HOH
hco2h ( formic acid )
Molecules non polar
HBrO ( Hypobromous acid )
SiF4 (Silicon tetrafluoride)
CH3CH3 (Ethane)
N2 (Nitrogen)
CO3 2- (Carbonate)
Heptane-
XeO4 (Xenon tetroxide)
CH3I (Methyl iodide)
CBr4 (Carbon tetrabromide)
AlCl3 (Aluminum trichloride)
NH4Br
C2H4Cl2 (dichloroethane)
ligroin
SO4 2- (Sulfate)
SF6 (Sulfur hexafluoride)
C2F2 ( Ethyne )
PO4 3- (phosphate)
GeH4 (Germane)
PBr5 (Phosphorus pentabromide)
NH4 (ammonium)
wax
CI4 (Tetraiodomethane)
bbr3 (BORON TRIBROMIDE)
IF4-
BeF2 (Beryllium difluoride)
GaH3 (Gallane)
SeBr4 (Selenium tetrabromide)
KrF4
C2H6 (Ethane)
O2 (Oxygen)
biphenyl
PO4 3
P4 (Phosphorus tetramer)
SO4 2-
naphthalene
XeCl2
BrCl5
SeF6
SeO3
SiH4 (silane)
carbon tetrabromide
AsF5 (Arsenic pentafluoride)
BeH2 (Beryllium hydride)
CH2
cholesterol
pentane
ClF2
C3H8
Propane
cyclohexane
chlorine
PF5 (Pentafluorophosphorane)
BrCl
CO2 (carbon dioxide)
CCl4 (CARBON TETRACHLORIDE)
XeF2
CS2 (CARBON DISULFIDE)
SO3 (SULFUR TRIOXIDE)
XeF4 (Xenon tetrafluoride)
BCl3 (BORON TRICHLORIDE)
BeCl2 (Beryllium dichloride)
CF4 (CARBON TETRAFLUORIDE)
Cl2 (Chlorine)
i3-
carbon tetrachloride
carbon disulfide
methane
glycine
sulfur trioxide
benzophenone
oxygen
AlF3 (Aluminum fluoride)
beryllium dichloride
C8H18 (octane)
C2Cl4
PF6-
XeCl4
SbF5 (ANTIMONY PENTAFLUORIDE)
CH3CH2CH3
C5H12 (PENTANE)
silane
s8
BF4
BF4-
SeCl6
BeBr2
BeI2
CSe2
Pcl4
C3H6
AlH3 (Aluminum trihydride)
TeO3 (Tellurium trioxide)
Br-Br
AsCl5
octane
carbonate ions
ClF4 plus
acids
kerosene
acetylene
C-C
ethane
CCl
Cl4
cbr
sulfate
butter
triglycerides
grease
beryllium chloride
carbon tetrafluoride
butane
silicon tetrafluoride
nitrogen
ICl2-
bromine
hydrocarbon
HE
boron trichloride
CS
sulfur hexafluoride
sis2
xenon tetrafluoride
CH4
vitamin e
Br2 (Bromine)
KNO3 ( Potassium nitrate )
C6H14 or CH3(CH2)4CH3 ( HEXANE )
C6H6 ( benzene )
I2 ( Iodine )
H2 ( Hydrogen )
SiO2 ( silica gel or silicon dioxide )
SiCl4 ( Silicon tetrachloride )
C2H4 ( Ethylene )
C2H2 (Ethyne)
KrF2 ( krypton difluoride )
toluene ( C7H8 )
F2 ( Fluorine )
SiBr4 ( silicon tetrabromide )
BH3 ( Borane )
BF3 ( Boron trifluoride )
xeo2 ( Xenon dioxide )
TeO3 ( Tellurium trioxide )
bcl2
PbCl2 ( Lead(II) chloride )
HgCl2 ( Mercury(II) chloride )
C2Br2 ( Dibromoacetylene )
CI2 or cl-cl ( Chlorine )
scl4i2
ch2i2 ( Diiodomethane )
if2-
n2f2
scl4f2
oil
Lipids
ICl4-
c ( carbon )
diethyl ether ( (C2H5)2O or CH3CH2OCH2CH3 )
soap
tryptophan
Proline
Phenylalanine
Alanine
Cysteine
Isoleucine
Leucine
Methionine
Valine
paraffin wax
C-H ( carbon hydrogen )
vegetable oil
Gasoline
H-H
F-F
FCN ( Cyanogen fluoride )
ClCN ( CYANOGEN CHLORIDE )
AlBr3 ( ALUMINUM BROMIDE )
(CH3CH2)2O
Petroleum Ether
C2F6
stearic acid
1-decanol
octahedral
C6H12
ch3ch2ch2ch2ch3
square planar
Helium ( He )
ibuprofen
propene
hcch
PF2Cl3
C3H4
Na2CO3 ( sodium carbonate )
phosphorus triodide ( PI3 )
C4H8 ( Isobutene )
vitamin a
SbCl5 ( Antimony pentachloride )
polyethylene
SnCl4 ( Tin(IV) chloride )
graphite
FeCl3 ( Iron (III) Chloride )
polystyrene
C2Cl6
decane
Sii4 ( Silicon tetraiodide )
boron trihydride
C2F4 ( Tetrafluoroethylene )
octanol
teflon
Ionic
NaBr
NaF
kf
NaNO3
cao
ki
lif
licl (LITHIUM CHLORIDE)
ammonium sulfate
MgF2
ki & lif
KCl ( Potassium Chloride )
MgBr2 ( Magnesium bromide )
CaF2 ( Calcium fluoride )
Na2O ( Sodium Oxide )
CsCl ( Cesium Chloride )
MgSO4 ( Magnesium Sulfate )
CuSO4 ( Copper (II) Sulfate )
CsI ( Cesium Iodide )
RbCl ( Rubidium chloride )
We get answers from
Resources:
https://en.wikipedia.org/wiki/Chemical_polarity
http://www.school-for-champions.com/chemistry/polar_molecules.htm#.WZIGddJJbcc
reference.com
www.quora.com
answers.yahoo.com
answers.com
youtube.com
google.com
https://pubchem.ncbi.nlm.nih.gov
If the answer is wrong, please comment in below article !
Question = Is I2 ( Iodine ) polar or nonpolar ?
Answer = I2 ( Iodine ) is Nonpolar